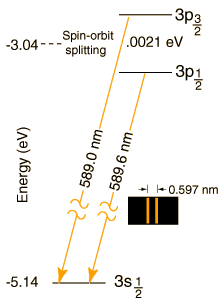
The 589.0 nm spectral line of the sodium yellow doublet is a transition from the 2P3/2
excited states to the 2S1/2 ground state of the sodium atom.
Energy level diagram showing how the two energy levels are split in the
presence of a weak magnetic field:
The 3P3/2 level is split because of L-S coupling.
The selection rules for this transition are:
Δ l = ±1
Δ j = ±1, 0
For the transition from 3P3/2 ===> 3S1/2, Δ l = +1 (since S = 0 and P = 1)
and Δ j = 0 for the 3P1/2 ===> 3S1/2 and +1 for the 3P3/2 ==> 3S1/2 transition. (j = s+l and is given by the subscript).
Therefore the transitions satisfy the selection rules.
No comments:
Post a Comment